X Chromosome Inactivation: A New Hope for Genetic Diseases
X chromosome inactivation is a fascinating process crucial to maintaining genetic balance in females, who possess two X chromosomes compared to males with only one. This unique mechanism ensures that one copy of the X chromosome is silenced, preventing an overabundance of gene expression. Understanding X chromosome inactivation is not just an academic pursuit; it holds the key to treating several genetic diseases, particularly X-linked disorders such as Fragile X syndrome and Rett syndrome. Recent discoveries on chromosomal silencing reveal potential therapeutic avenues that aim to “unsilence” these critical genes, paving the way for innovative treatments. By unlocking the mysteries of X-inactivation, researchers aim to provide hope for those affected by debilitating genetic conditions, transforming the landscape of modern medicine.
The process known as X chromosome inactivation, often referred to as XCI, plays a vital role in gene regulation, particularly in females who have a double dose of X-linked genes. This phenomenon, also described as chromosomal silencing, is essential for preventing gene dosage imbalance between sexes, ensuring that one X chromosome is functionally inactive. Insights into XCI mechanisms could lead to breakthrough therapies for neuromuscular and neurodevelopmental conditions, such as Fragile X syndrome therapy and Rett syndrome treatment. As scientific research unfolds, the potential to exploit this chromosomal regulation not only enhances our understanding of human genetics but also offers promising interventions for various genetic disorders. Embracing these advanced applications can revolutionize therapeutic approaches and improve the quality of life for individuals affected by X-linked conditions.
Understanding X Chromosome Inactivation: Mechanisms and Implications
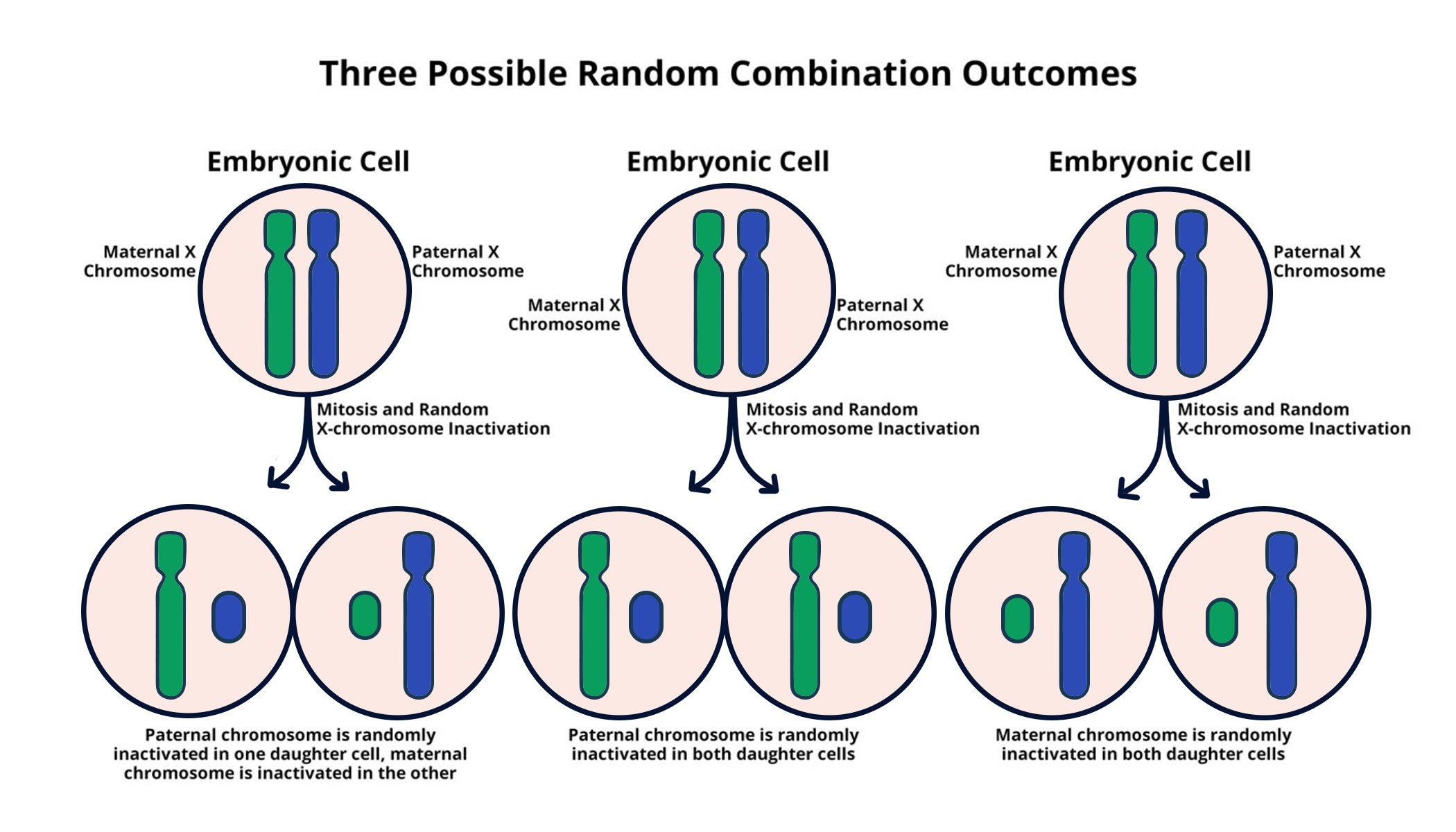
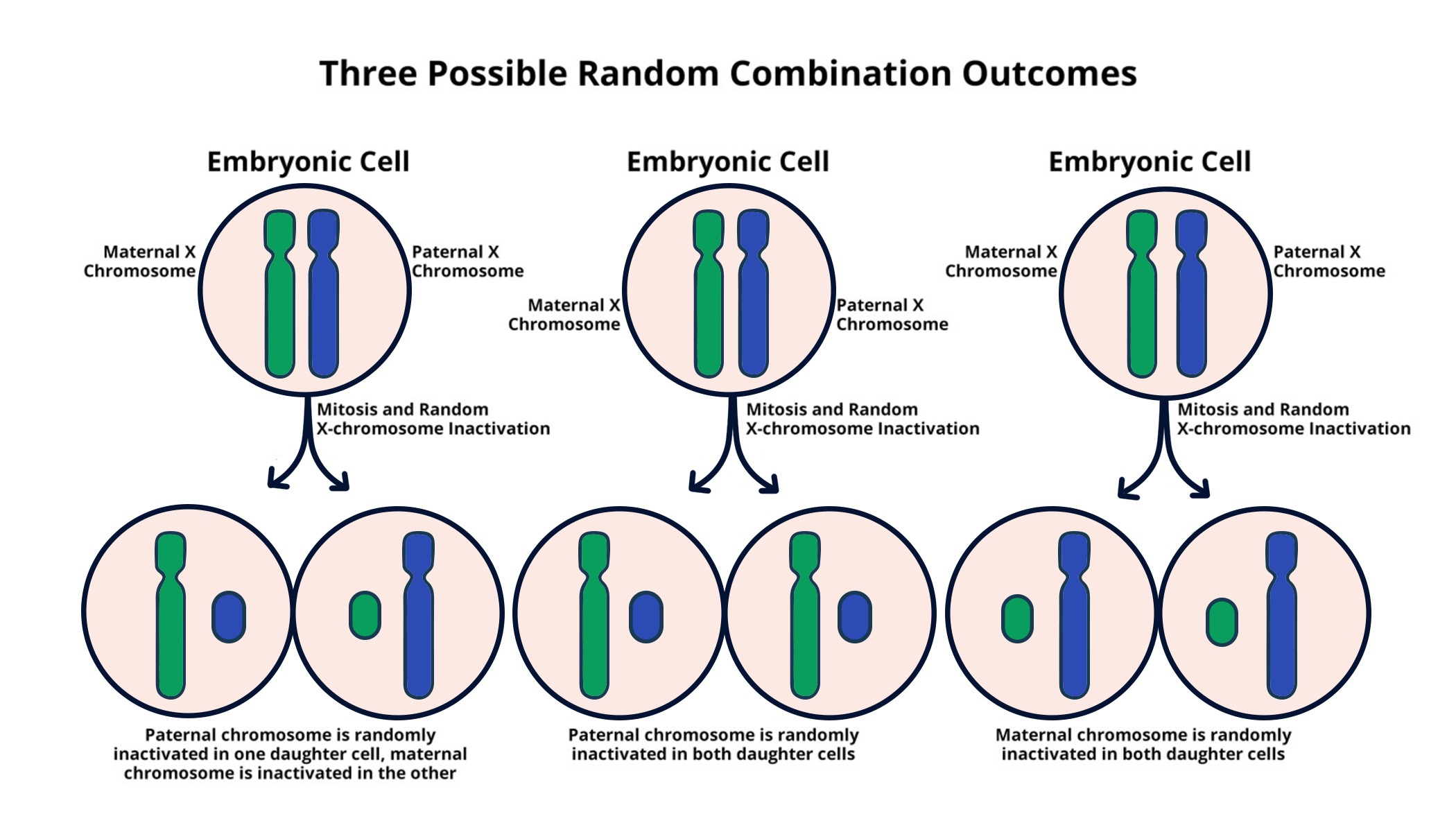
X chromosome inactivation (XCI) is a crucial process in the development of females, where one of the two copies of the X chromosome is silenced to prevent excess gene dosage. This fascinating biological mechanism is not just a matter of genetic balance; it plays a fundamental role in the expression of X-linked disorders. In contrast to males, who possess a single X chromosome, females have the unique ability to randomly inactivate one of their X chromosomes, ensuring that cells function properly despite the potential for genetic disparities. Understanding the intricate processes that govern XCI gives insight into how genetic diseases such as Fragile X Syndrome and Rett Syndrome manifest and can be treated.
Recent research by Jeannie Lee’s lab has shed light on the biophysical processes involved in this silencing. Using a “Jell-O” like substance, the lab illustrates how Xist RNA interacts with the chromosomal material to orchestrate the inactivation of one X chromosome in females. This chromosomal silencing is not merely a whimsical analogy; it is a vital part of cellular operation that influences genetic expression and susceptibility to X-linked disorders.
The implications of understanding X chromosome inactivation extend beyond basic science. The capacity to manipulate XCI represents a potential breakthrough for treating genetic diseases. These discoveries have prompted significant interest in therapies that could reverse the inactivation process, thereby allowing healthy copies of genes on the inactive X chromosome to be expressed. This therapeutic approach opens avenues for treating conditions traditionally thought to be incurable, such as Fragile X syndrome, which affects cognitive development and is linked to mutations on the X chromosome. Researchers are now distancing themselves from traditional strategies, aspiring to unsilence these genes and restore their function in affected individuals.
Advancements in Treatments for X-Linked Disorders
Recent scientific advancements suggest that we may be on the brink of effective treatments for X-linked disorders like Fragile X syndrome and Rett syndrome. These conditions are primarily caused by mutations in genes located on the X chromosome, rendering the development of effective therapies particularly challenging. Understanding X chromosome inactivation has revealed pathways and methods to ‘unsilence’ these mutated genes, potentially allowing the affected individuals to produce functional proteins once again.
The Lee lab’s findings about Xist and its role in chromosomal silencing have paved the way for innovative therapy design. Initial studies have demonstrated that by targeting the ‘Jell-O’ substance enveloping the X chromosome, researchers can selectively activate genes that were previously silent due to XCI. This approach is groundbreaking, as it not only holds promise for females with X-linked disorders but also offers therapeutic potential for males, whose X-linked mutations could also be corrected through the same unsilencing strategies.
As clinical trials begin to take shape, the anticipation for accessible treatments for genetic diseases is palpable. Lee notes that the next steps involve optimizing these novel compounds and conducting safety studies, integral to ensuring that these therapies do not inadvertently cause unintended effects on non-targeted genes. The goal is to create a clinical framework for the administration of these therapies to provide relief to those suffering from debilitating X-linked disorders. By understanding how to manipulate X chromosome inactivation, scientists are devising targeted therapies that could significantly improve the quality of life for those affected by genetic conditions.
The Role of Chromosomal Silencing in Genetic Disease
Chromosomal silencing, particularly in the context of X chromosome inactivation, holds significant implications for the field of genetic diseases. It serves as a mechanism that can either exacerbate or mitigate the effects of mutations present on the X chromosome. This nuanced understanding of how genes are expressed based on chromosomal configuration allows scientists to explore new avenues for disease treatment and management. In cases of X-linked conditions like Fragile X syndrome, where a mutation may be present on one X and a healthy version on the other, chromosomal silencing becomes pivotal.
Understanding the dynamics of XCI offers crucial insights into why some genes remain silent while others can be expressed, even after efforts to ‘unsilence’ them. The Lee lab’s exploration of the interaction between Xist and chromosomal material elucidates the processes behind this silencing, suggesting that certain X-linked mutations could have therapeutic targets. As researchers delve deeper into understanding these relationships, they not only uncover the complexities of genetic diseases but also lay the groundwork for potential treatments that could change lives.
Moreover, the study of chromosomal silencing encourages a re-evaluation of existing therapeutic strategies for managing genetic disorders. Traditional approaches to treating X-linked disorders have primarily focused on managing symptoms rather than targeting the underlying genetic issues. With the advancements in understanding XCI and its implications, there is a shift towards more innovative approaches that aim to correct genetic expression at the source. This paradigm shift holds the promise of more personalized medicine, where treatments are tailored to the individual’s unique genetic makeup and specific mutations, paving the way for more effective interventions in the management of X-linked disorders.
Exploring Future Directions in Genetic Disease Treatment
As research on XCI progresses, the potential for developing effective treatments for various genetic disorders appears increasingly feasible. The ability to unsilence genes on the X chromosome not only provides hope for those affected by conditions like Fragile X syndrome and Rett syndrome but also opens up new avenues for understanding a broader range of genetic diseases. Future studies will likely focus on expanding the applications of these findings to other genetic conditions connected to chromosomal silencing.
Furthermore, the excitement surrounding these advancements has sparked interest in interdisciplinary collaboration among geneticists, molecular biologists, and clinical researchers. By combining insights from various fields, teams are better positioned to develop comprehensive approaches to genetic disease treatment. As Lee’s lab continues to refine these techniques and prepare for clinical trials, it exemplifies the potential for groundbreaking discoveries to translate into impactful therapies that could revolutionize the landscape of genetic disease management.
Additionally, public support and funding are crucial in advancing this promising area of research. As more people become aware of the implications of X chromosome inactivation, advocacy for funding initiatives to explore potential treatments intensifies. This supportive environment not only encourages scientific innovation but also fosters engagement among patients and families affected by these diseases, which is essential for driving research efforts forward. The future direction of genetic disease treatment hinges on this synergy of science, support, and advocacy, ensuring that knowledge gained from understanding X chromosome inactivation paves the way for tangible outcomes that can change lives for the better.
Frequently Asked Questions
What is X chromosome inactivation and why is it important for genetic diseases?
X chromosome inactivation is a process that occurs in female mammals where one of the two X chromosomes is silenced to ensure that females, like males, have a single functional copy of X-linked genes. This is crucial for preventing an overload of gene expression and has significant implications for genetic diseases linked to X chromosome mutations, such as Fragile X syndrome and Rett syndrome. Understanding this process is key for developing targeted therapies for these X-linked disorders.
How does X chromosome inactivation relate to Rett syndrome treatment?
The process of X chromosome inactivation is particularly relevant for Rett syndrome, as the gene mutations causing the disorder are often found on the X chromosome. Therapies targeting the unsilencing of the inactive X chromosome may allow access to healthy gene copies, potentially offering a treatment path for Rett syndrome. This approach aims to restore normal gene function in affected cells.
Can X chromosome inactivation mechanisms aid in Fragile X syndrome therapy?
Yes, the mechanisms behind X chromosome inactivation offer promising avenues for Fragile X syndrome therapy. Researchers like Jeannie Lee are investigating ways to unsilence the X-linked genes associated with Fragile X syndrome, potentially allowing the expression of healthy genes that may have been silenced due to mutations. This could lead to novel treatments that address the underlying genetic issues.
What is chromosomal silencing and how does it affect genetic diseases?
Chromosomal silencing, particularly X chromosome inactivation, is a regulatory mechanism that prevents the expression of genes on one of the two X chromosomes in females. This silencing is essential for maintaining balanced gene dosage but can complicate conditions like X-linked disorders. When a disease-causing mutation exists on a silenced X chromosome, therapies aimed at reopening these chromosomes could provide relief from genetic diseases by restoring gene function.
Are there potential therapies emerging from research on X chromosome inactivation?
Recent research on X chromosome inactivation, particularly by Jeannie T. Lee’s lab, has suggested that compounds can be developed to unsilence inactivated X chromosomes. These therapies hold the potential to treat genetic diseases such as Fragile X and Rett syndromes by allowing the expression of healthy gene copies located on the inactive X chromosome, marking a significant breakthrough in how we approach X-linked disorders.
Why is X chromosome inactivation a focus in genetic research?
X chromosome inactivation is a major focus in genetic research because it plays a critical role in gene regulation in females and is directly implicated in numerous genetic diseases. Understanding this complex process is vital for developing targeted therapies for X-linked disorders, enhancing our ability to treat conditions like Fragile X syndrome and Rett syndrome effectively.
How might freeing inactivated X chromosomes impact treatment outcomes for genetic disorders?
Freeing inactivated X chromosomes could dramatically improve treatment outcomes for genetic disorders by allowing cells to utilize the healthy gene versions that are currently silenced. This restoration of gene function is expected to have minimal impact on healthy genes, suggesting that targeted therapies might alleviate symptoms with fewer side effects, potentially offering new hope for patients with conditions such as Fragile X syndrome and Rett syndrome.
| Key Points |
|---|
| The X chromosome poses a challenge for cells due to females having two copies and males having only one, requiring one X to be inactivated in females. |
| Jeannie Lee at Harvard Medical School has been pivotal in uncovering the mechanisms behind X chromosome inactivation. |
| Inactivation involves a gelatinous substance that coats chromosomes, playing a crucial role in maintaining their structure and functionality. |
| The gene Xist is crucial for initiating the inactivation process by altering the properties of the surrounding ‘Jell-O’. |
| Research indicates potential therapies for genetic diseases such as Fragile X and Rett syndromes by unsilencing inactivated X chromosomes. |
| The Lee lab’s ongoing work aims to optimize therapeutic approaches and initiate clinical trials for these genetic disorders. |
| Mysteries remain about why freed mutated genes function well while healthy genes remain largely unaffected, indicating an intriguing capacity limitation within the cell. |
Summary
X chromosome inactivation is a critical biological process that enables female cells to manage their two X chromosomes effectively. This process not only maintains genetic balance but also opens up new avenues for treating genetic diseases linked to X chromosome mutations. With the pioneering research led by Jeannie Lee, there’s hope for groundbreaking therapies for conditions like Fragile X and Rett syndromes. As science progresses, understanding and manipulating X chromosome inactivation could transform the landscape of genetic disorder treatments, providing much-needed relief to affected individuals and families.